What's the Science Behind Our Medical Research & Clinical Trials?
Dr. Brian Czerniecki and his team are leading the research effort in the science of immunotherapy. Their vaccine for breast cancer patients has a higher efficacy than other treatments on the market in Clinical Phase I and Phase II. Phase III trials are in the final planning stages.
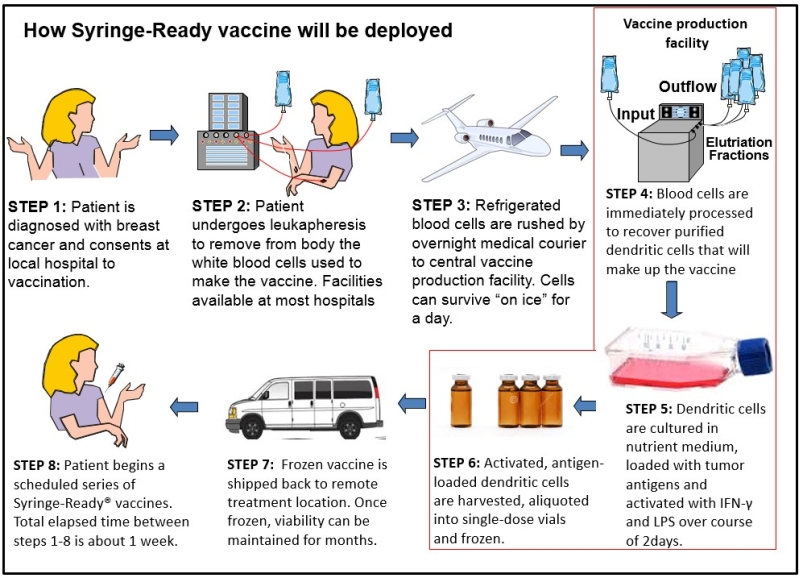
The following diagram was created by Dr. Gary Koski to portray how the production of the personalized ImmunoRestoration Syringe-Ready Vaccine (Ir-Vax) interfaces with patients, the manufacturing facility, primary care physicians, and medical oncologists.
After a patient is diagnosed with breast cancer and consents to receiving the vaccination, they undergo leukapheresis, which is a clinical procedure that removes blood from a patient and separates it into three different constituents: red blood cells, plasma, and white cells. The red blood cells and plasma are then returned to the patient while the white cells are kept to make the Ir-Vac. The white cells are then shipped, overnight, to a central vaccine production facility.
White blood cells are a collection of different immune system cells, each with distinct physical and functional characteristics. Only one type of white blood cell is needed for the Ir-Vax production. This cell is called a “monocyte”, and it comprises about 15 percent of all white blood cells. At the vaccine production facility, the white blood cells will undergo elutriation. The elutriation procedure separated the monocytes from the other white blood cells and purifies them to levels greater than 90 percent.
Dendritic cells are another type of white blood cell that develop from monocytes. These cells are the “guards” of the immune system. They patrol the body looking for biochemical signs of microbial infection. When these are encountered, the dendritic cells acquire proteins, or “antigens” from these microorganisms, break them down into smaller pieces, decorate their own cell surface with these fragments, and present them to other white blood cells called T lymphocytes, which will normally lead the fight against the bacteria, viruses, or parasites. The T cells will multiply and go throughout the body on a search-and-destroy mission against any target that is marked with these antigen fragments (i.e., the infecting microorganism). This is how our bodies routinely eliminate microbial threats. The Ir-Vax is specifically designed to mimic this natural process of microbial elimination and direct it instead against cancer.
The reason the immune system usually fails to destroy cancer on its own is because there are not enough differences between a normal, healthy cell and a cancerous cell to indicate a threat and trigger the elimination process.
“I know of no vaccine therapy for early breast or any other cancer that can make the disease disappear entirely in a substantial proportion of subjects in the 4-6 weeks prior to scheduled surgery. Dr. Czerniecki has treated over 80 patients since 2004, and in addition to these complete responders, he is seeing a strong apparent suppression of disease recurrence in the treated patients. All this is observed in the absence of any serious side effects."
To produce the Ir-Vac the monocytes are cultured overnight in a nutrient medium containing the cytokines Interleukin-4 and GM-CSF. These instruct the monocytes to become dendritic cells. The next day a highly purified cell wall component from bacteria called lipopolysaccharide (LPS) and synthetic pieces (peptides) of HER-2 cancer protein are added to the culture. The presence of LPS is one of the main signals dendritic cells use to detect infection. Therefore, the pretense of the bacterial LPS in the culture tricks the dendritic cells into behaving as though the HER-2 fragments are coming from a dangerous bacterial infection that requires immediate action. The cytokine IFN-y is also added to the dendritic cell culture, which, in conjunction with the LPS, causes the dendritic cells to produce soluble factors, including Interleukin-12, which can instruct the T cells to become particularly destructive to tumors.
A few hours after the dendritic cells receive these activating signals, they are collected and frozen in individual, single-dose vials. Samples of the vaccine are then put through a rigorous quality control/quality assurance evaluation before release. Once frozen, the dendritic cells can be kept for months. The frozen vaccine can then be shipped back to the patient at a treatment site remote from the manufacturing center.
Another innovation of Ir-Vax is the Syringe Ready aspect of the vaccine. For traditional cell-based therapy agents, freezing of the cells requires the presence of high concentrations of a cryopreservative compound (like dimethyl sulfoxide DMSO)) that prevents cells from being damaged by the formation of ice crystals during freezing. The larger amounts of cryopreservatives must be washed out of the preparation before they can be safely injected into the patient.
According to FDA regulations, if such a washing step is performed, a second round of quality control/quality assurance must be performed immediately prior to the injection in order to ensure that the vaccine was not adulterated during the process. This requires specialized facilities and protocols at the treatment site which can greatly add to costs. The proprietary Syringe Ready process uses much lower amounts of cryopreservatives, so low in fact that it is not necessary to remove them prior to injection. Once thawed, the Ir-Vac is used just as any injectable drug or vaccine. It is simply drawn into a hypodermic syringe and directly injected into the patient.
Once inside the patient, the HER-2 peptide-coated dendritic cells seek out T lymphocytes to inform them that the body is under attack by a dangerous infection that can be distinguished from healthy bodily cells by the presence of the HER-2 peptides. The T lymphocytes then begin to divide and form a small army of cells programmed to attack anything bearing the HER-2protein.
The additional Ir-Vax single-dose vials are thawed and injected into the patient on a dose schedule that is usually once a week for 4-6 weeks followed by booster injections separated by several months. With each introduction of the vaccine, the immune response is amplified, and the T cells are kept on high-alert status until the threat is eliminated.
ImmunoRestoration believes that time is of the essence to manage Dr. Czerniecki’s patents with good stewardship, which will benefit numerous breast cancer patients.